Introduction: Chronic lymphoproliferative disorder of natural killer cells (CLPD-NK) is a rare form of large granular lymphocytic leukemia. The clinical characteristics and outcomes of patients (pts) with CLPD-NK in the literature are limited. We aim to describe the patient characteristics, laboratory features, and clinical outcomes of CLPD-NK by reporting on, to our knowledge, the largest single-center cohort of CLPD-NK in the United States.
Methods: We retrospectively identified Mayo Clinic pts with CLPD-NK, defined per the WHO 5 th edition and 2022 International Consensus Classification. We conducted chart review to collect clinical and laboratory data. Overall survival (OS) was plotted using Kaplan-Meier. Time to first treatment (TTFT) was displayed using cumulative incidence methodology accounting for competing risk of death. Univariable Cox regression models investigated associations between patient characteristics and outcomes (OS and TTFT).
Results: We identified 45 pts who were diagnosed with CLPD-NK between 1997 and 2022. The median age at diagnosis was 69 years (range: 25-90) and 32 pts (71%) were male. At the time of diagnosis, 10 pts (22%) had a concomitant autoimmune disorder and 9 (20%) had laboratory evidence of a serologic abnormality (monoclonal gammopathy, rheumatoid factor, etc.). In addition, 10 pts (22%) had a prior diagnosis of a hematologic malignancy and 12 (27%) had a prior diagnosis of a solid tumor. Clinically, 4 pts (9%) had B symptoms, 15 (33%) had splenomegaly, and 13 (29%) were transfusion dependent.
At diagnosis, median hemoglobin (hgb) was 10.2 g/dL, with 25 pts (56%) showing a hgb <11g/dL and 8 (18%) demonstrating a hgb <8 g/dL. The median platelet count was 202x10^9/L, with 12 pts (27%) demonstrating a platelet count <150x10^9/L. The median white blood cell count was 5.8x10^9/L. The median absolute neutrophil count (ANC) was 1.2x10^9/L, with 29 pts (64%) demonstrating an ANC <1.5x10^9/L. The median absolute lymphocyte count (ALC) was 3.8x10^9/L, with 20 pts (44%) demonstrating an ALC >4x10^9/L. The median absolute abnormal NK cell count per flow cytometry was 2.2x10^9/L.
Flow cytometry revealed killer cell immunoglobulin-like receptor (KIR) restriction in all pts, with 27 (61%) lacking KIR expression and 17 (39%) demonstrating KIR restriction in CD158a, CD158b, and/or CD158e. CD7 expression was present in 42 pts (93%; 31% dim/partial), CD8 expression was present in 22 (49%; 42% d/p), CD56 expression was present in 17 (39%; 23% d/p), CD57 expression was present in 26 (59%; 46% d/p), CD94 expression was present in 33 (77%; 7% d/p), and NKG2A expression was present in 21 (50%; 5% d/p). Bone marrow examination (n=30) revealed that the median number of NK infiltrate was 11% (range: 5-40%) of cellularity, with 20 demonstrating an intrasinusoidal infiltrative pattern.
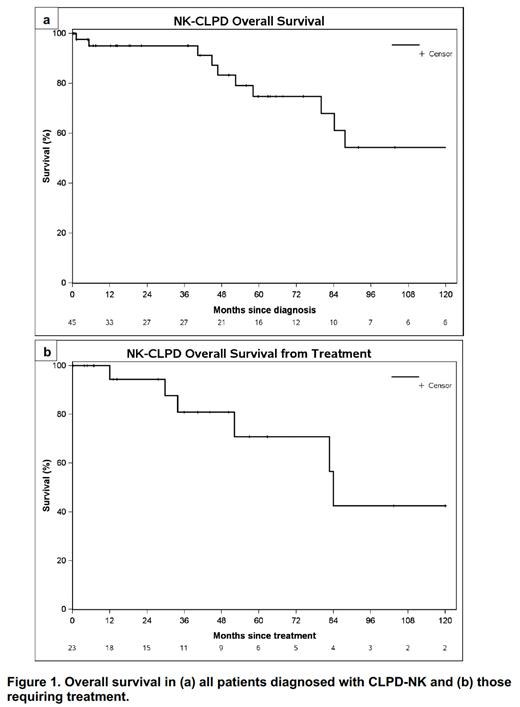
The median follow-up was 41 months (mo), and 23 pts required at least one line of treatment. Treatment indications included anemia (39%), neutropenia (4%), bi/pancytopenia (39%), lymphocytosis (4%), or other (13%). The median TTFT was 32 mo (95% CI 2-93 mo). Hgb <11g/dL was associated with a shorter TTFT (9.7 vs 187 mo); however, neutropenia and thrombocytopenia were not associated with TTFT. For first-line treatment, in addition to frequent steroid use, 16 pts (70%) received a methotrexate-based regimen, 6 (26%) received a cyclophosphamide-based regimen, and 1 (4%) received a cyclosporine-based regimen. The median TTFT was not reached. Of those who required systemic treatment, 9 pts required a second-line therapy. The median OS from diagnosis was 162 mo (95% CI: 84-not estimable, NE), with 33 pts alive at last follow-up (Figure 1a). Increasing age (HR 1.2, 95% CI 1.1-1.3, p=0.002) and lack of CD94 expression (HR 5.6, 95% 1.3-25.0, p=0.02) were associated with worse OS. In the 23 pts who required treatment, the median OS from treatment initiation was 84 mo (95% CI: 52-NE) (Figure 1b).
Conclusions: In this large cohort of 45 pts, the majority had neutropenia and anemia, and approximately half required systemic treatment. Estimated median OS from diagnosis exceeded a decade for the entire cohort and was seven years after treatment initiation. Anemia was associated with a shorter TTFT, while increasing age and lack of CD94 expression were associated with worse OS. This rare disease warrants additional investigations to understand optimal management and improve outcomes, especially for those requiring treatment.
Disclosures
Kenderian:Kite/Gilead: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Torque: Consultancy; Lentigen: Research Funding; CapstanBio: Consultancy, Other: Scientific advisory board; Novartis: Consultancy, Honoraria, Patents & Royalties, Research Funding, Speakers Bureau; Mettaforge: Patents & Royalties; LEAHLabs: Consultancy, Current equity holder in private company, Research Funding; Morphosys: Research Funding; Tolero/Sumtomo: Research Funding; Sendero: Patents & Royalties; Humanigen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties, Research Funding, Speakers Bureau; Juno/BMS: Other: Membership on an entity's board of directors or advisory committees, Research Funding; MustangBio: Patents & Royalties; Luminary therapeutics: Other: scientific advisory board . Kay:Agios Pharm: Membership on an entity's Board of Directors or advisory committees; Vincerx: Research Funding; Sunesis: Research Funding; Behring: Membership on an entity's Board of Directors or advisory committees; Bristol Meyer Squib / Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Beigene: Membership on an entity's Board of Directors or advisory committees; Genentech: Research Funding; Pharmcyclics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees; Dren Bio: Membership on an entity's Board of Directors or advisory committees; Astra Zeneca: Membership on an entity's Board of Directors or advisory committees; Dava Oncology: Membership on an entity's Board of Directors or advisory committees; Acerta Pharma: Research Funding; Abbvie: Membership on an entity's Board of Directors or advisory committees, Research Funding; boehringer ingelheim: Membership on an entity's Board of Directors or advisory committees; Juno Therapeutics: Membership on an entity's Board of Directors or advisory committees. Wang:Incyte: Membership on an entity's Board of Directors or advisory committees, Research Funding; Eli Lilly: Membership on an entity's Board of Directors or advisory committees, Research Funding; LOXO Oncology: Membership on an entity's Board of Directors or advisory committees, Research Funding; Innocare: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; BeiGene: Membership on an entity's Board of Directors or advisory committees; Kite: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Genmab: Research Funding; TG Therapeutics: Membership on an entity's Board of Directors or advisory committees; Novartis: Research Funding; Morphosys: Research Funding; Genentech: Research Funding; Astra Zeneca: Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy. Parikh:Vincerx: Research Funding; AstraZeneca: Membership on an entity's Board of Directors or advisory committees; Agios Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Accerta Pharmaceuticals: Research Funding; Bristol Myers Squibb-Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie Inc: Membership on an entity's Board of Directors or advisory committees, Research Funding; Dava Oncology: Membership on an entity's Board of Directors or advisory committees; Genentech: Research Funding; Boehringer Ingelheim Pharmaceuticals Incc: Membership on an entity's Board of Directors or advisory committees; Sunesis: Research Funding; CSL Behring: Membership on an entity's Board of Directors or advisory committees; Beigene: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Juno Therapeutics: Membership on an entity's Board of Directors or advisory committees; Dren Bio: Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Membership on an entity's Board of Directors or advisory committees, Research Funding. Ding:Merck: Consultancy, Honoraria, Research Funding; BeiGene: Consultancy, Honoraria, Research Funding; MEI pharama: Consultancy, Honoraria; Alexion: Consultancy, Honoraria; Octapharma: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AstraZeneca: Research Funding; AbbVie: Research Funding; DTRM: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal